
The carbon and oxygen forms triple bond and two electrons of oxygen and four electrons of oxygen involve in a bonding orbital. More electronegative element has a partial negative charge on it and other gains slightly positive charge.Ĭarbon monoxide has one carbon and one oxygen atoms having a total of 10 valence electrons. This is mainly because of difference in their electronegativity. Whereas the bond in which unequal charge distribution exists on both atoms of a molecule are polar bond. The bond in which equal sharing of electrons is done by both atoms is a nonpolar bond because, in such molecules, charge density on both atoms is the same resulting in a nonpolar molecule. This bond can be polar or nonpolar depending upon the atoms involved. In a covalent bond, two atoms share the pair of electrons to stabilize each other and remain bonded with each other.
In this bond, oppositely charged atoms stabilize each other and connected by a strong bond known as an ionic bond. The bond through which two oppositely charged atoms are linked with each other is an ionic bond. The bonds that are strongest out of these and largely present among chemical compounds are ionic and covalent.
There are several types of bonds like ionic, metallic, covalent, and hydrogen. The atoms are held together through bonds. The polarity of molecules is dependent upon different factors that are discussed in the below topic. Unequal charge distribution occurs on both atoms of the molecule of carbon monoxide.Ĭonclusion What are Polar and Nonpolar Molecules? The direction of dipole moment originates towards Carbon atom ie C←O. In this way, polarisation occurs among carbon and oxygen atoms. In the formation of a triple bond in CO molecule, 4 electrons come from oxygen and 2 electrons from the carbon atom.Īs a result, in one bonding orbital two electrons are involved from oxygen atom that forms a dipolar bond. The bond length in the CO molecule is around 12 pm.Ĭarbon and oxygen atoms both constitute the 10 valence electrons. Out of three bonds, two of them are pi bonds and one is sigma bond. The Carbon and Oxygen atoms are attached via a triple bond. The molecule of Carbon Monoxide consists of one carbon atom and one oxygen atom. It is toxic to animals that carry hemoglobin in their blood because the CO molecules attach to the hemoglobin that carries oxygen. It is itself a flammable gas by nature and inhaling Carbon monoxide gas of concentrations greater than 35 ppm can cause illness and other health problems too.ĬO gas is slightly lesser dense than air. The carbon and oxygen atom have unequal charge distribution and therefore CO bond has a net dipole moment making CO a polar molecule.Ĭarbon monoxide is produced in ou environment through various means like the burning of wood, gasoline, charcoal, and other fuel. So, Is CO Polar or Nonpolar? CO (Carbon monoxide) is polar in nature because of the difference in electronegativity of carbon (2.55) and oxygen (3.44) atoms. So, I will answer this question and also discuss the surrounding topics too. Many students may also have doubts about whether CO is polar or not. This is produced in many ways like burning of wood, charcoal, gasoline. B., and thanks for watching.Carbon monoxide denoted by its chemical formula as CO is colorless, tasteless, and odorless gas present in the environment. So that's the Lewis structure for CO, carbon monoxide.
#Co carbon monoxide lewis structure full#
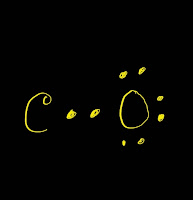
So by now we've used all the valence electrons, all 10, and each of the atoms in the Lewis structure for CO has a full outer shell-has an octet, with 8 valence electrons. Now the Oxygen has 8, but the Carbon has 8 as well. We'll move two more of these valence electrons to form a triple bond between the Carbon and the Oxygen. The Oxygen still has 8, but now the Carbon has 6. Let's take these two from Oxygen and share them with the Carbon. But the Carbon only has 4 valence electrons. We can see Oxygen has 8 valence electrons, so it has an octet. We'll put 2 electrons between the atoms to form a chemical bond, that's 2 and then around the outer atoms, that's 4, 6, 8, and 10. So we have a Carbon and an Oxygen atom bonded together. We have 4 valence electrons for Carbon and 6 for Oxygen, for a total of 10 valence electrons. Transcript: This is the CO Lewis structure: Carbon monoxide.


 0 kommentar(er)
0 kommentar(er)
